Supply for Organic Rhodiola Rosea Extract Factory for Slovenia
Supply for Organic Rhodiola Rosea Extract Factory for Slovenia Detail:
[Latin Name] Rhodiola Rosea
[Plant Source] China
[Specifications] Salidrosides:1%-5%
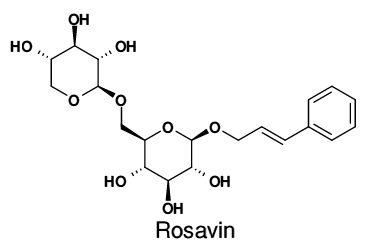
Rosavin:3% HPLC
[Appearance] Brown fine powder
[Plant Part Used] Root
[Particle size] 80 Mesh
[Loss on drying] ≤5.0%
[Heavy Metal] ≤10PPM
[Storage] Store in cool & dry area, keep away from the direct light and heat.
[Package] Packed in paper-drums and two plastic-bags inside.
[What is Rhodiola Rosea]
Rhodiola Rosea (also known as Arctic root or golden root) is a member of the family Crassulaceae, a family of plants native to the arctic regions of Eastern Siberia. Rhodiola rosea is widely distributed in Arctic and mountainous regions throughout Europe and Asia. It grows at altitudes of 11,000 to 18,000 feet above sea level.
There are numerous animal and test tube studies showing that rhodiola has both a stimulating and a sedating effect on the central nervous system; enhance physical endurance; improves thyroid, thymus, and adrenal function; protects the nervous system, heart and liver; and has antioxidant and anticancer properties.
[Function]
1 Enhancing immunity and delaying aging;
2 Resisting radiation and tumor;
3 Regulating nervous system and metabolism, effectively limiting melancholy feeling and mood, and promoting mental status;
4 Protecting cardiovascular, dilating coronary artery,preventing coronary arteriosclerosis and arrhythmia.
Product detail pictures:

Related Product Guide:
We have been ready to share our knowledge of advertising and marketing worldwide and recommend you suitable products and solutions at most competitive price ranges. So Profi Tools supply you best benefit of money and we're ready to create with each other with Supply for Organic Rhodiola Rosea Extract Factory for Slovenia , The product will supply to all over the world, such as: Rome, Uzbekistan, Iraq, Many kinds of different products are available for you to choose, you can do one-stop shopping here. And customized orders are acceptable. Real business is to get win-win situation, if possible, we would like to provide more support for customers. Welcome all nice buyers communicate details of products with us!!
via YouTube Capture
Cardo Mariano Propiedades; en este video descubre las Propiedades y Beneficios del Cardo Mariano.
https://lasdietassaludables.com/
Es Cardo Mariano esutilizado como protector del hígado y lo desintoxica de sustancias nocivas como el alcohol y las drogas.
*****************************
Otro video relacionado de la serie hierbas medicinales:
Suscribete a nuestro Canal (Subscribe):
https://www.youtube.com/user/LaHierbasMedicinales
Encuentranos en FacebooK:
https://www.facebook.com/ladietasaludable
Comparte nuestro video:
********************
El Cardo Mariano es una Hierba bianual de gran tamaño que llega hasta los 2 m de altura sus hojas son grandes con manchas de color blanco en su superficie, lobulados y con contorno espinoso. El cardo mariano es conocido por ser beneficioso para el hígado, ya que le protege de las toxinas estimulando el crecimiento de nuevos tejidos. Esta protección es importante para eliminar los venenos biológicos.
Videos relacionados:
cardo mariano para el hígado
contraindicaciones del cardo mariano
propiedades del diente de leon
propiedades del cardo mariano contraindicaciones
propiedades del cardo mariano en gotas
propiedades del cardo mariano en capsulas
propiedades del cardo mariano wikipedia
propiedades del cardo mariano en infusion
cardo mariano higado
cardo mariano para adelgazar
silymarin
propiedades del cardo mariano para el higado
silybum marianum
plantas para desintoxicar el higado
This is the first business after our company establish, products and services are very satisfying, we have a good start, we hope to cooperate continuous in the future!