Europe style for St John’s wort extract Factory in Brazil
Europe style for St John’s wort extract Factory in Brazil Detail:
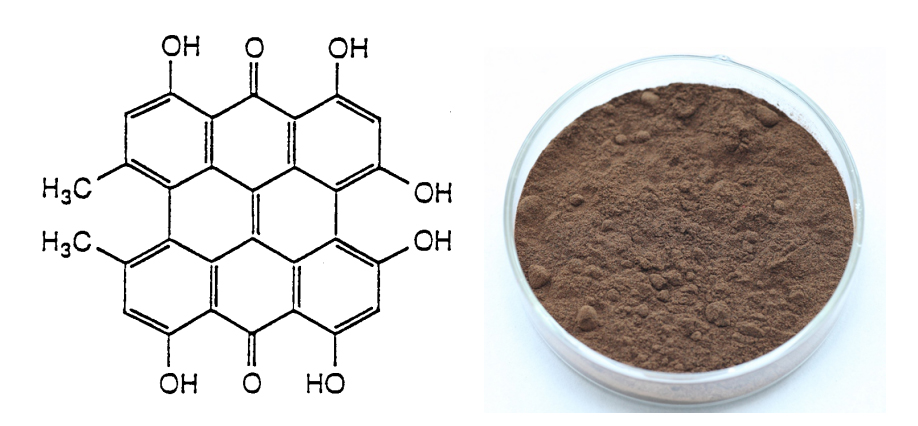
[Latin Name] Hypericum perforatum
[Plant Source] From China
[Appearance] Brown fine powder
[Specifications] 0.3% Hypericin
[Particle size] 80 Mesh
[Loss on drying] ≤5.0%
[Heavy Metal] ≤10PPM
[Pesticide residue] EC396-2005, USP 34, EP 8.0, FDA
[Storage] Store in cool & dry area, keep away from the direct light and heat.
[Package] Packed in paper-drums and two plastic-bags inside.
[What is St. John's wort]
St. John’s wort (Hypericum perforatum) has a history of use as a medicine dating back to ancient Greece, where it was used for a range of illnesses, including various nervous disorders. St. John’s wort also has antibacterial, antioxidant, and antiviral properties. Because of its anti-inflammatory properties, it has been applied to the skin to help heal wounds and burns. St. John’s wort is one of the most commonly purchased herbal products in the United States.
In recent years, St. John’s wort has been studied extensively as a treatment for depression. Most studies show that St. John’s wort may help treat mild-to-moderate depression, and has fewer side effects than most other prescription antidepressants.
[Functions]
1. Anti-depressive and sedative properties;
2. Effective remedy for the nervous system, relaxing tension, and anxiety and lifting the spirits;
3. Anti-inflammatory
4. Improve capillary circulation
Product detail pictures:

Related Product Guide:
Sticking for the perception of "Creating products of top quality and producing friends with people today from all around the world", we constantly place the desire of shoppers to start with for Europe style for St John’s wort extract Factory in Brazil , The product will supply to all over the world, such as: Romania, Thailand, Cape Town, Our products are mainly exported to Europe, Africa, America, the Middle East and Southeast Asia and other countries and regions. We have enjoyed a great reputation among our customers for quality products and good services.We would make friends with businessmen from at home and abroad, following the purpose of "Quality First, Reputation First, the Best Services."
Rock Steady is an all natural male and female sex enhancment capsule which can be ordered by calling (305) 304-8122
The company leader recept us warmly, through a meticulous and thorough discussion, we signed a purchase order. Hope to cooperate smoothly